Unit 6 worksheet 3 ionic compounds – Embark on a captivating journey into the realm of ionic compounds as we delve into Unit 6 Worksheet 3. Prepare to unravel the mysteries of these fascinating substances, exploring their formation, properties, and myriad applications in our world.
From the basics of naming ionic compounds to understanding their unique physical and chemical characteristics, this worksheet promises an engaging exploration into the world of chemistry.
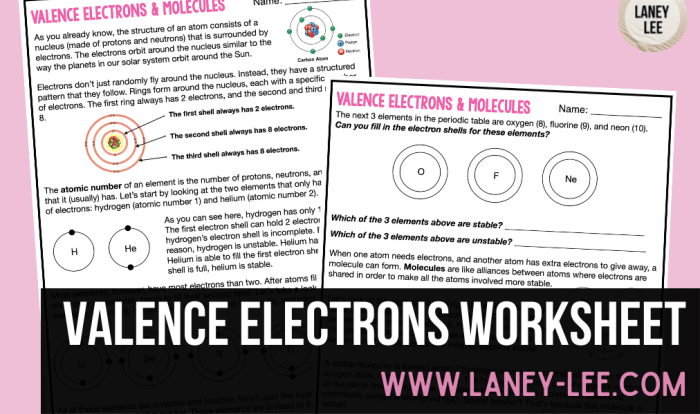
Ionic Compounds Overview

Ionic compounds are formed when a metal loses one or more electrons to a nonmetal. The resulting positively charged metal ion is attracted to the negatively charged nonmetal ion, forming an ionic bond. Ionic compounds are typically hard, brittle, and have high melting points.
Examples of Ionic Compounds
Some common examples of ionic compounds include:
- Sodium chloride (NaCl)
- Potassium chloride (KCl)
- Calcium fluoride (CaF 2)
- Magnesium oxide (MgO)
Naming Ionic Compounds
Ionic compounds are formed when a metal loses one or more electrons to a nonmetal. The resulting ions have opposite charges and are attracted to each other by electrostatic forces.
The Stock system is a systematic way of naming ionic compounds. According to this system, the name of the cation (the positively charged ion) is written first, followed by the name of the anion (the negatively charged ion). The charge of the cation is indicated by a Roman numeral in parentheses after the cation’s name.
For example, the ionic compound NaCl is named sodium chloride. The sodium ion has a charge of +1, and the chloride ion has a charge of -1. Therefore, the name of the compound is sodium(I) chloride.
Here is a table with some additional examples of ionic compounds and their correct names:
| Formula | Name |
|---|---|
| KCl | Potassium chloride |
| CaO | Calcium oxide |
| Fe2O3 | Iron(III) oxide |
| CuSO4 | Copper(II) sulfate |
Properties of Ionic Compounds
Ionic compounds possess distinctive physical and chemical properties that stem from the strong electrostatic forces between their oppositely charged ions. These properties include solubility, melting point, and electrical conductivity, each of which plays a crucial role in various applications.
Solubility
Ionic compounds generally exhibit high solubility in polar solvents, such as water. This solubility is attributed to the strong interaction between the polar solvent molecules and the ions of the compound. The solvent molecules surround and solvate the ions, weakening the electrostatic forces holding them together and allowing them to dissolve.
The solubility of ionic compounds in water can vary significantly. Some compounds, such as sodium chloride (NaCl), dissolve readily, while others, such as calcium sulfate (CaSO 4), have limited solubility. This variation is influenced by factors like ion size, charge, and the hydration energy of the ions.
Melting Point
Ionic compounds typically have high melting points compared to molecular compounds. The strong electrostatic forces between the ions require a substantial amount of energy to overcome, resulting in a high melting point. For example, sodium chloride has a melting point of 801°C, while water has a melting point of 0°C.
The high melting points of ionic compounds make them suitable for applications where high-temperature resistance is required, such as in crucibles and refractory materials.
Electrical Conductivity
Ionic compounds are generally good conductors of electricity in both the molten and aqueous states. In the molten state, the ions are free to move and carry charge, allowing the compound to conduct electricity. In aqueous solutions, the ions dissociate and become surrounded by solvent molecules, enabling them to conduct electricity.
The electrical conductivity of ionic compounds is utilized in various applications, including batteries, fuel cells, and electrolytes.
Writing Chemical Formulas for Ionic Compounds: Unit 6 Worksheet 3 Ionic Compounds
Writing chemical formulas for ionic compounds involves understanding the charges of the ions involved and balancing their charges to form a neutral compound.
To write the chemical formula for an ionic compound, follow these steps:
- Identify the cation (positively charged ion) and the anion (negatively charged ion) that form the compound.
- Determine the charges of the ions using the periodic table or the rules for common ions.
- Balance the charges of the ions by adjusting the subscripts of the ions in the formula. The subscripts represent the number of ions of each type in the compound.
- Write the chemical formula using the symbols of the ions and the balanced subscripts.
Here are some examples of ionic compounds and their chemical formulas, highlighting the charges of the ions:
| Ionic Compound | Chemical Formula | Cation Charge | Anion Charge |
|---|---|---|---|
| Sodium chloride | NaCl | +1 | -1 |
| Magnesium oxide | MgO | +2 | -2 |
| Calcium fluoride | CaF2 | +2 | -1 |
| Potassium iodide | KI | +1 | -1 |
| Aluminum sulfide | Al2S3 | +3 | -2 |
Balancing Chemical Equations Involving Ionic Compounds
Chemical equations are symbolic representations of chemical reactions that depict the changes in the composition and structure of reactants and products. Balancing chemical equations is crucial to ensure that the number of atoms of each element remains the same on both sides of the equation, adhering to the law of conservation of mass.
When dealing with ionic compounds, balancing chemical equations requires a slightly different approach.
To balance chemical equations involving ionic compounds, follow these steps:
Step 1: Write the Unbalanced Equation
Write the chemical equation for the reaction, including the chemical formulas of the reactants and products. Do not worry about balancing at this stage.
Step 2: Separate Ionic and Molecular Compounds
Identify the ionic compounds in the equation. Ionic compounds are typically formed between a metal and a non-metal. Separate the ionic compounds from the molecular compounds (compounds that do not contain metal ions).
Step 3: Write the Ionic Equations
For each ionic compound, write the dissociation equation, which shows the compound separating into its constituent ions. Write the dissociation equations below the unbalanced equation.
Step 4: Balance the Ionic Equations
Balance the ionic equations by adjusting the coefficients in front of each ion. The goal is to ensure that the total charge on both sides of the equation is equal.
Step 5: Combine the Balanced Ionic Equations
Combine the balanced ionic equations to obtain the balanced chemical equation. Add the coefficients from the ionic equations to the coefficients in front of the ionic compounds in the original equation.
Step 6: Check for Balance, Unit 6 worksheet 3 ionic compounds
Finally, check if the balanced chemical equation is balanced by counting the number of atoms of each element on both sides. The number of atoms of each element should be the same on both sides.
Applications of Ionic Compounds
Ionic compounds are essential in various industries, playing crucial roles in medicine, food processing, and construction.
Medicine
*
-*Sodium chloride (NaCl)
Common salt, essential for electrolyte balance and nerve function.
-
-*Potassium chloride (KCl)
Used as a potassium supplement and to treat electrolyte imbalances.
-*Calcium carbonate (CaCO3)
Antacid, helps neutralize stomach acid.
Food Processing
*
-*Sodium chloride (NaCl)
Enhances flavor and acts as a preservative.
-
-*Potassium nitrate (KNO3)
Curing agent in meat preservation.
-*Sodium benzoate (C6H5COONa)
Preservative in food and beverages.
Construction
*
-*Calcium sulfate (CaSO4)
Gypsum, used in drywall and plaster.
-
-*Sodium carbonate (Na2CO3)
Used in glass and cement production.
-*Magnesium oxide (MgO)
Fire-resistant material used in building materials.
Question Bank
What are ionic compounds?
Ionic compounds are formed when atoms transfer electrons to achieve a stable electron configuration, resulting in positively charged ions (cations) and negatively charged ions (anions) that attract each other to form a crystal lattice.
How do you name ionic compounds?
Ionic compounds are named using the Stock system, where the cation is named first, followed by the anion. The charge of the cation is indicated using Roman numerals in parentheses.
What are the properties of ionic compounds?
Ionic compounds typically have high melting and boiling points, are soluble in water, and conduct electricity when dissolved or molten.