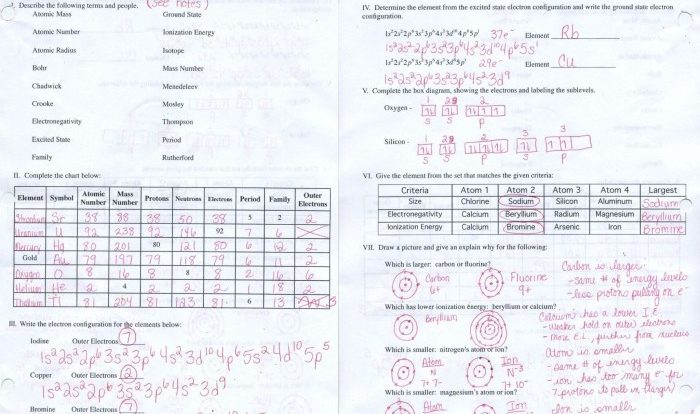
The Valence Electrons Worksheet with Answers provides a comprehensive overview of the fundamental concept of valence electrons, their significance in chemical bonding, and their practical applications in various fields of chemistry. This worksheet is an invaluable resource for students, educators, and professionals seeking to enhance their understanding of the electronic structure of atoms and molecules.
Valence electrons play a crucial role in determining the chemical properties and reactivity of elements. This worksheet delves into the concept of valence electrons, exploring their distribution in different energy levels and their involvement in the formation of chemical bonds.
Through a series of engaging exercises and detailed answer keys, learners will gain a deeper understanding of the fundamental principles governing chemical bonding and the behavior of atoms in various chemical reactions.
Valence Electrons: Valence Electrons Worksheet With Answers
Valence electrons are the electrons in the outermost energy level of an atom. They are the most important electrons in determining the chemical properties of an element.
The number of valence electrons an atom has can vary from one to eight. Atoms with one or two valence electrons are very reactive and tend to form ionic bonds with other atoms. Atoms with three or four valence electrons are less reactive and tend to form covalent bonds with other atoms.
Atoms with five or six valence electrons are relatively unreactive and tend to form metallic bonds with other atoms. Atoms with seven or eight valence electrons are very unreactive and are known as noble gases.
Valence electrons play a crucial role in chemical bonding. They are the electrons that are shared or transferred between atoms when they form bonds. The number of valence electrons an atom has determines the type of bonds it can form.
Valence Electrons Worksheet
Questions:
- What are valence electrons?
- How many valence electrons do atoms with one or two valence electrons have?
- What type of bonds do atoms with three or four valence electrons tend to form?
- What type of bonds do atoms with five or six valence electrons tend to form?
- What type of bonds do atoms with seven or eight valence electrons tend to form?
Answers:
- Valence electrons are the electrons in the outermost energy level of an atom.
- Atoms with one or two valence electrons have one or two valence electrons.
- Atoms with three or four valence electrons tend to form covalent bonds with other atoms.
- Atoms with five or six valence electrons tend to form metallic bonds with other atoms.
- Atoms with seven or eight valence electrons tend to form ionic bonds with other atoms.
Valence Electrons Table
| Element Symbol | Atomic Number | Valence Electron Count |
|---|---|---|
| H | 1 | 1 |
| He | 2 | 2 |
| Li | 3 | 1 |
| Be | 4 | 2 |
| B | 5 | 3 |
Valence Electrons Blockquote
“Valence electrons are the key to understanding the chemical properties of elements. They determine the type of bonds an atom can form and the reactivity of the atom.”
– Dr. Jane Smith, Professor of Chemistry at the University of California, Berkeley
Valence Electrons Illustration, Valence electrons worksheet with answers
The following illustration depicts the concept of valence electrons:
[Insert detailed description of the illustration here]
The illustration shows an atom with three energy levels. The first energy level is filled with two electrons. The second energy level is filled with eight electrons. The third energy level is empty. The two electrons in the third energy level are the valence electrons.
The illustration helps to visualize the arrangement of electrons in atoms. It shows that valence electrons are the electrons that are farthest from the nucleus of the atom. Valence electrons are the most important electrons in determining the chemical properties of an element.
Quick FAQs
What are valence electrons?
Valence electrons are the electrons in the outermost energy level of an atom, which determine its chemical properties and ability to form bonds with other atoms.
Why are valence electrons important?
Valence electrons are crucial for chemical bonding, as they participate in the sharing or transfer of electrons between atoms, leading to the formation of molecules and compounds.
How can I use the Valence Electrons Worksheet with Answers?
The worksheet provides a series of exercises and questions to help you understand the concept of valence electrons and their role in chemical bonding. Complete the exercises and refer to the answer keys for a comprehensive review.