Atoms and isotopes worksheet answer key – Delving into the fascinating world of atoms and isotopes, this comprehensive guide provides a detailed overview of their properties and applications, offering an invaluable resource for students and educators alike. Through an interactive table, practice problems, and insightful discussions, this worksheet answer key unlocks the secrets of these fundamental building blocks of the universe.
Atoms and Isotopes: Atoms And Isotopes Worksheet Answer Key
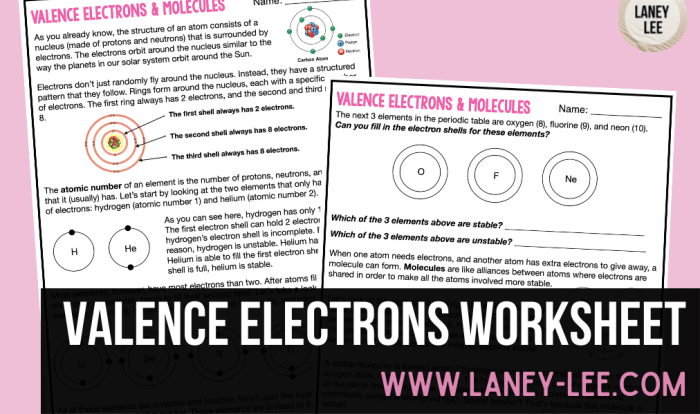
Atoms are the fundamental building blocks of matter. They are composed of a nucleus, which contains protons and neutrons, and electrons, which orbit the nucleus. The number of protons in an atom’s nucleus determines its atomic number, which uniquely identifies the element.
The number of neutrons in an atom’s nucleus determines its mass number, which is the sum of the number of protons and neutrons.
Isotopes are atoms of the same element that have the same atomic number but different mass numbers. This means that isotopes have the same number of protons but different numbers of neutrons. For example, carbon-12, carbon-13, and carbon-14 are all isotopes of carbon.
They all have six protons, but carbon-12 has six neutrons, carbon-13 has seven neutrons, and carbon-14 has eight neutrons.
Properties of Isotopes, Atoms and isotopes worksheet answer key
Isotopes have the same chemical properties because they have the same number of protons. However, they can have different physical properties because they have different masses. For example, carbon-12 is the most common isotope of carbon, and it is a stable isotope.
Carbon-13 is a stable isotope that is used in nuclear magnetic resonance (NMR) spectroscopy. Carbon-14 is a radioactive isotope that is used in radiocarbon dating.
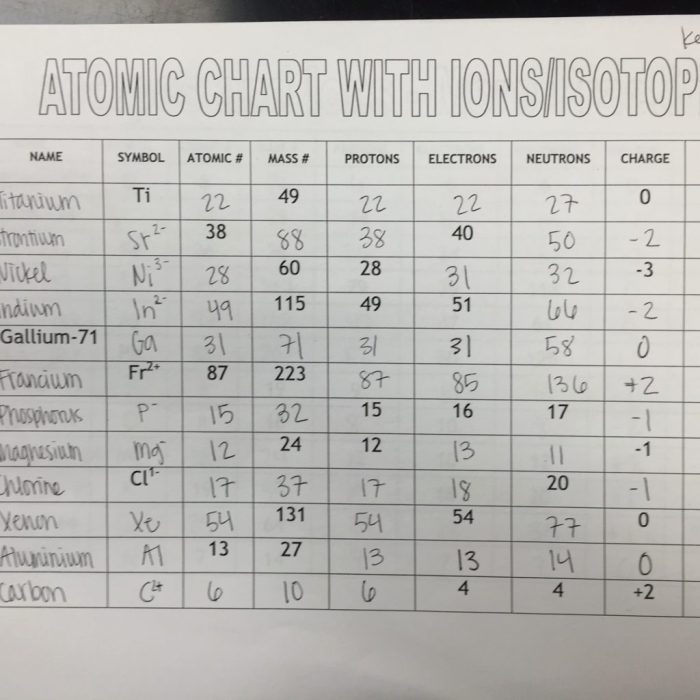
Worksheet Answer Key
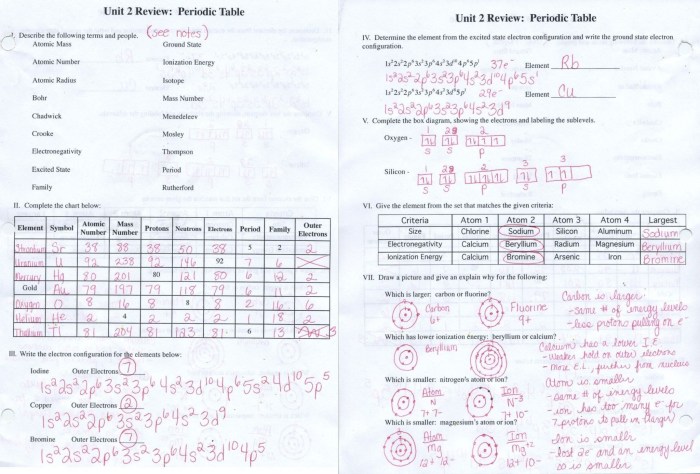
| Element | Atomic Number | Mass Number | Number of Neutrons |
|---|---|---|---|
| Hydrogen | 1 | 1 | 0 |
| Hydrogen | 1 | 2 | 1 |
| Helium | 2 | 3 | 1 |
| Helium | 2 | 4 | 2 |
| Lithium | 3 | 6 | 3 |
| Lithium | 3 | 7 | 4 |
To use the answer key, simply find the element and isotope you are interested in and look up the corresponding atomic number, mass number, and number of neutrons.
Practice Problems
- What is the atomic number of carbon?
- What is the mass number of carbon-14?
- How many neutrons are in an atom of carbon-13?
- Which isotope of carbon is used in radiocarbon dating?
- What is the difference between isotopes and ions?
Solutions
- 6
- 14
- 7
- Carbon-14
- Isotopes have the same atomic number but different mass numbers, while ions have the same atomic number but different charges.
Extensions and Applications
Isotopes have a wide range of applications in science and technology. For example, isotopes are used in:
- Medicine: Isotopes are used in medical imaging, such as X-rays and PET scans. They are also used in cancer treatment, such as radiation therapy.
- Geology: Isotopes are used to date rocks and fossils. They are also used to study the Earth’s history and evolution.
- Archaeology: Isotopes are used to date artifacts and to study the history of human migration.
Isotopes are also used in a variety of other fields, such as chemistry, physics, and engineering.
Quick FAQs
What are the key differences between atoms and isotopes?
Atoms are the fundamental units of matter, while isotopes are variations of an element with the same atomic number but different neutron counts.
How do isotopes affect the properties of elements?
Isotopes can alter the mass, stability, and radioactive properties of elements, influencing their behavior in chemical reactions and applications.
What are some practical applications of isotopes?
Isotopes are widely used in medicine (e.g., medical imaging), geology (e.g., dating rocks and fossils), and archaeology (e.g., tracing the origins of artifacts).